Somatic Cell Genome Editing (SCGE) Platform
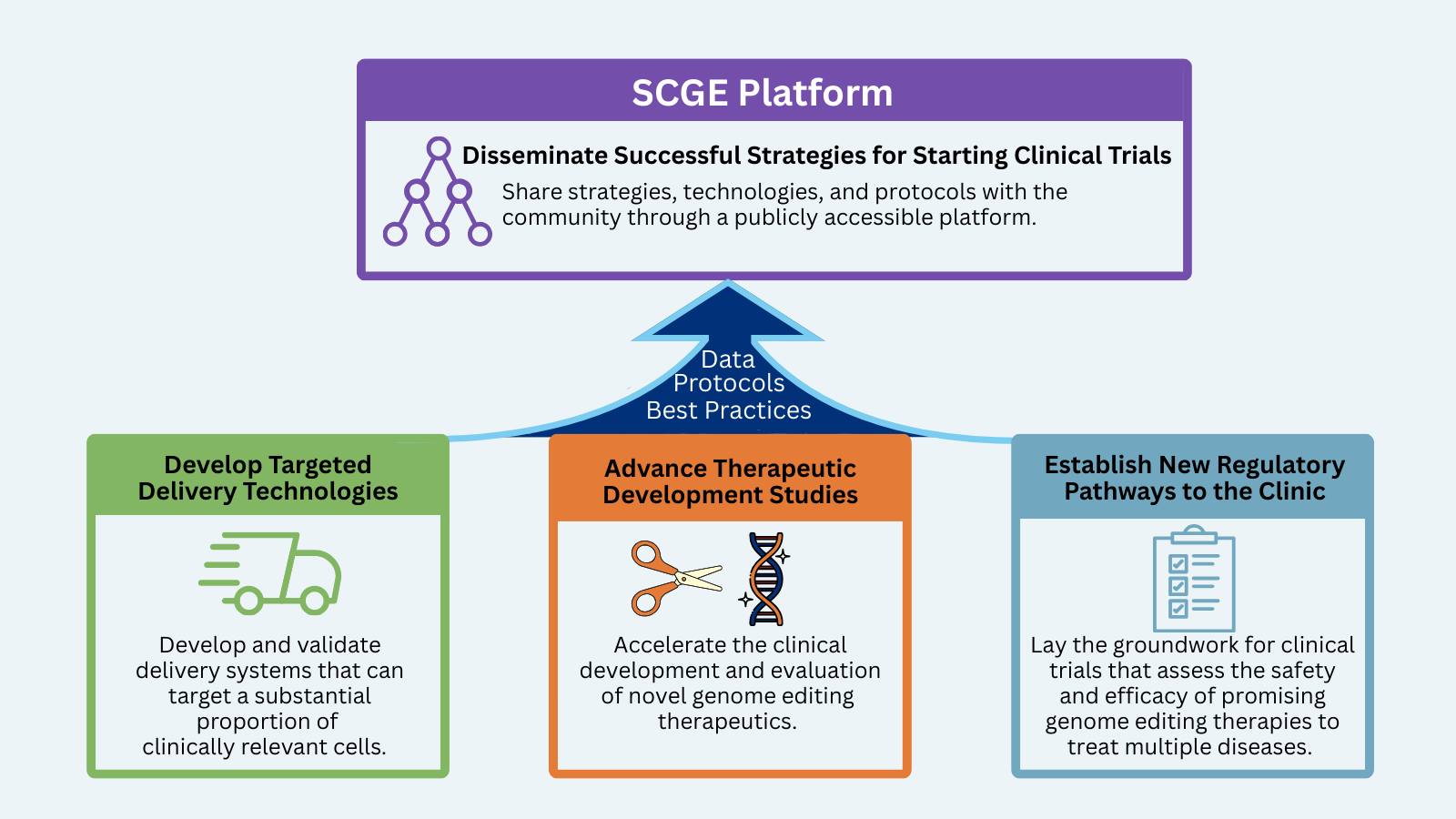
The National Institutes of Health (NIH) is leading an effort to help accelerate genome editing approaches from the lab to the clinic through the Somatic Cell Genome Editing (SCGE) Program. Phase I of the program included the SCGE Toolkit to disseminate the outcomes focused on translating safe and effective genome editing therapeutics into the clinic. During Phase 2 the Translational Coordination and Dissemination Center (TCDC) will support and work cooperatively with all initiatives, projects, and members to facilitate the objectives of the entire SCGE Consortium and the broader gene editing therapy field through development of the SCGE Platform, consisting of multiple initiatives.
- The SCGE Platform is tracking and curating information on current and past gene therapy and gene editing clinical trials to help investigators leverage other successful trials
- Explore the Gene Therapy Trial Browser
- The Platform will help investigators accelerate their efforts to get their therapies to the clinic by:
- Collecting and collating useful resources and form templates specific to gene editing therapies and products to help investigators work with and submit Investigational New Drug (IND) applications to the Federal Drug Administration (FDA)
- Learning and communicating best practices regarding a successful IND application
- Collecting and sharing data and protocols generated by consortium laboratories
SCGE Platform Tools & Resources
Gene Therapy Trial Browser
Explore our comprehensive database of current and past gene therapy and gene editing clinical trials.
Explore TrialsSCGE Regulatory Documents
Access pre-IND briefing books, FDA responses, and regulatory guidance documents.
View DocumentsProjects
Explore Phase 2 IND-enabling studies and collaborative research projects in genome editing.
View Projects