Program Snapshot
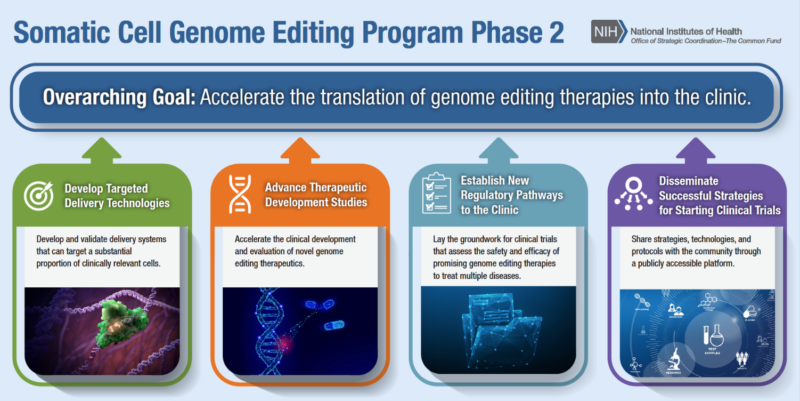
The goal of the SCGE program is to accelerate the development of genome editing therapies into the clinic. For Phase 2, there are four program initiatives:
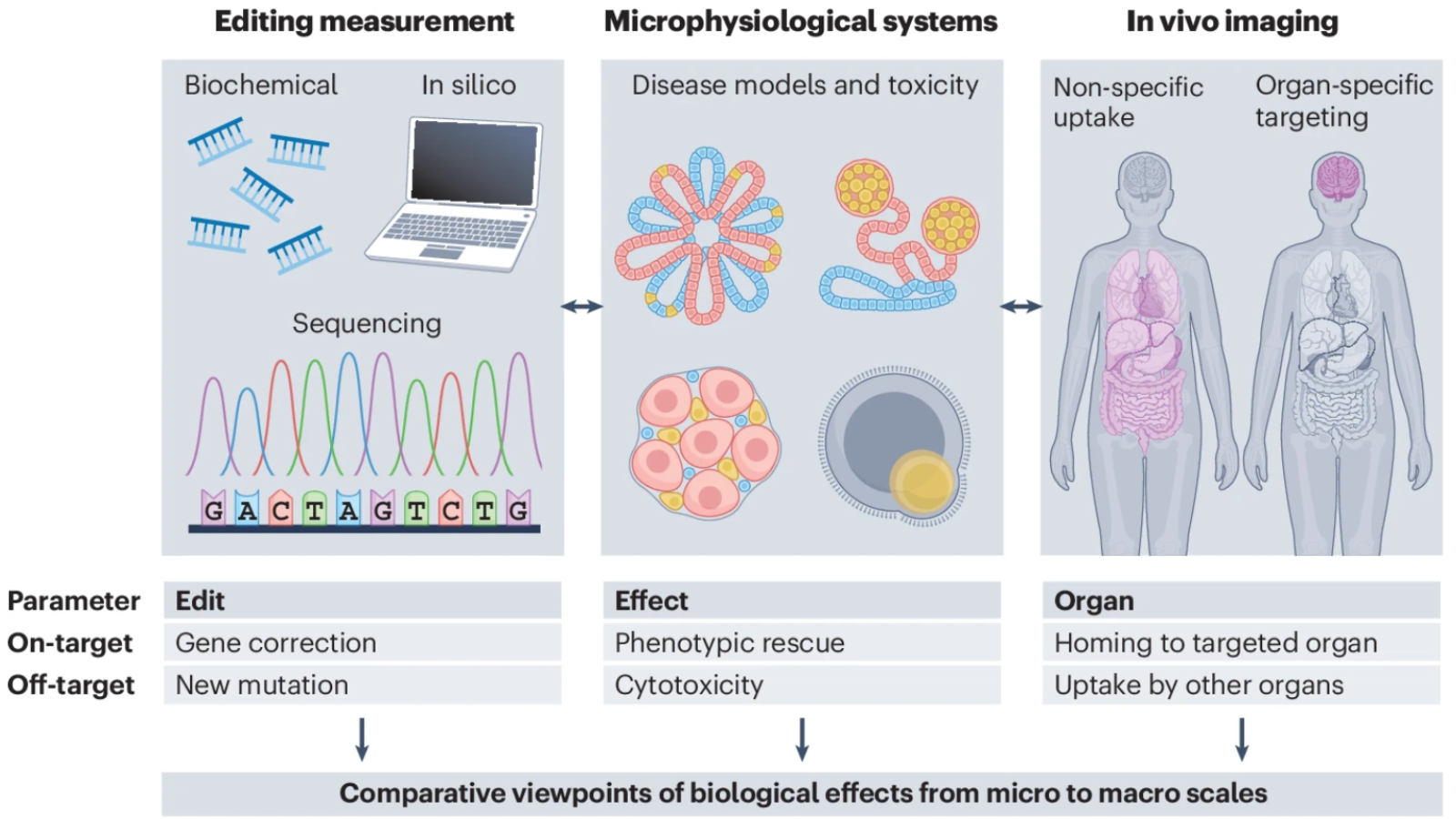
- Developing technologies and assays for safety and efficacy studies
- Optimizing genome editing-based therapeutic leads to support advancement towards clinical trials
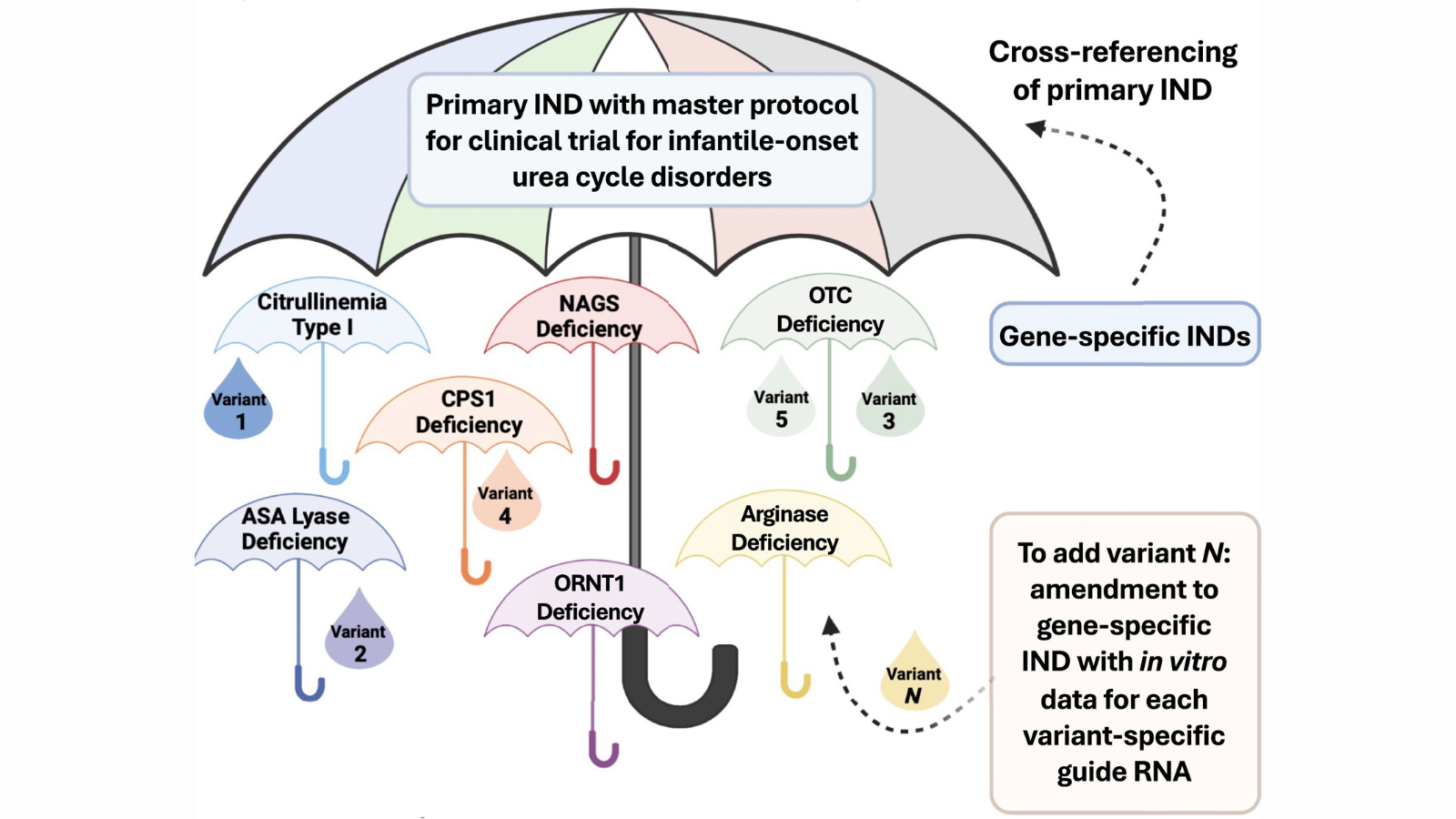
- Supporting novel genome editing clinical trials for more than one disease
- Fostering collaboration and sharing new technologies and protocols with the public and research community
For ethical, legal, and safety reasons, the SCGE program does NOT support any research activities on genome editing in reproductive (germ) cells.
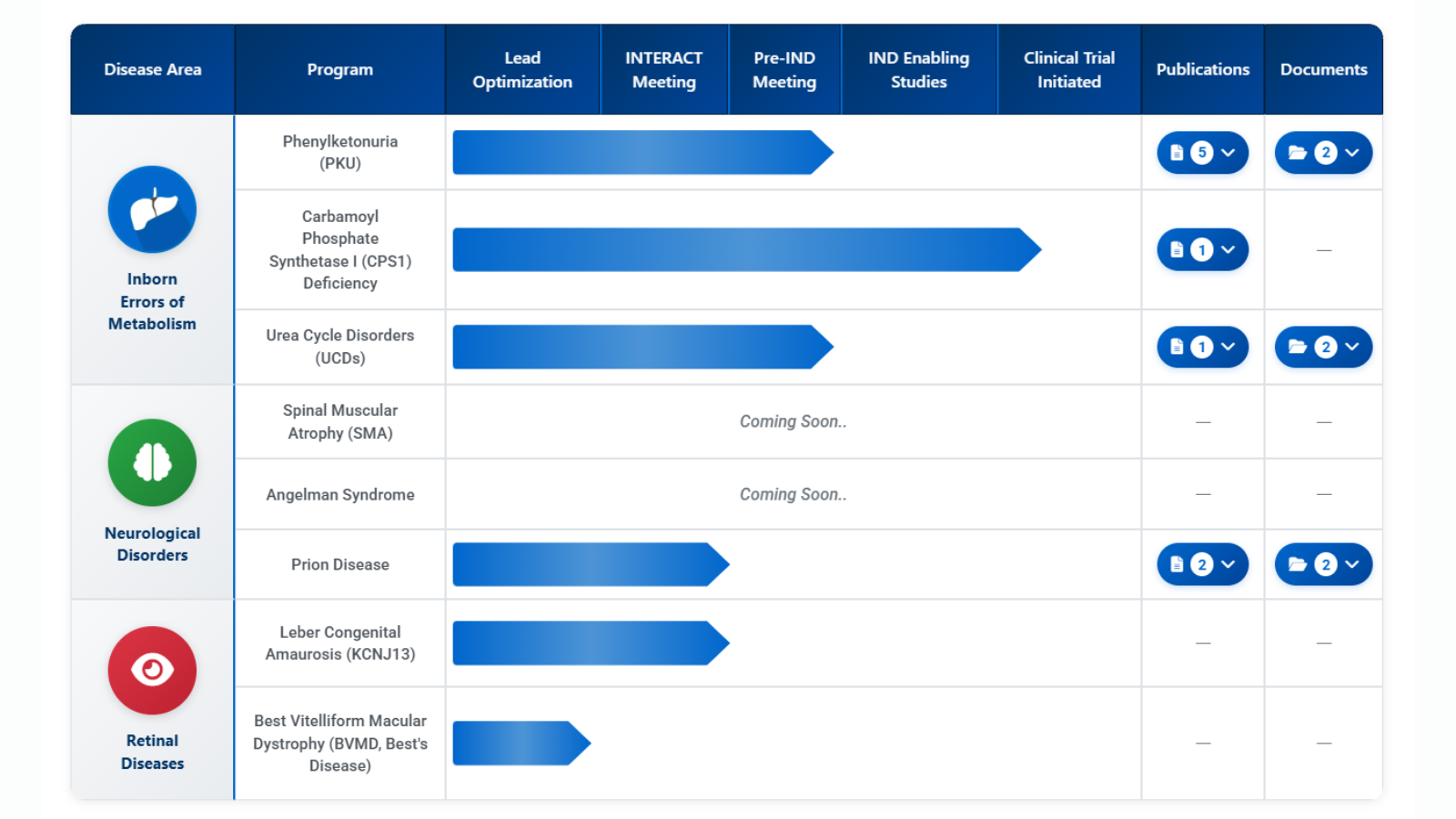
Learn more about individual and collaborative research projects, as well as the SCGE program and deliverables, below: