Information Guide for Rare Disease Patients and Caregivers
Navigating a rare disease is challenging for patients and caregivers. We’ve created this page as a compilation of general information on rare diseases, links to educational and financial resources, education on clinical trials and care team members, and more.
Keep reading to learn more, or find a PDF of this information at this link.
Rare Disease Overview

- The definition of what “rare disease” means varies by country and/or region
- USA: Affects fewer than 200,000 Americans
- Europe: Affects fewer than 1 in 2,000 people
- World Health Organization (WHO): Affects fewer than 65 in 100,000 people
- There have been over 7,000 rare diseases identified to date
- It’s estimated that rare diseases impact 25-30+ million people in the United States, and over 300 million people worldwide
- Around 70% of rare diseases start in childhood
- Genetics are thought to be a factor in many rare diseases
- Many rare diseases don’t have effective treatments yet
Resources for Patients & Caregivers
NORD Rare Disease Database: Database of rare diseases with information including symptoms, treatments, support resources, patient advocacy groups, and more
Genetic and Rare Diseases Information Center (GARD): Provides free access to reliable, easy-to-understand information about rare diseases
PAN Foundation Disease Funds: Disease-specific grants that can cover things such as medication copays, insurance premiums, transportation, and more
RareCare- NORD’s Patient Assistance Programs: Financial assistance for things such as medication, insurance premiums and co-pays, diagnostic testing, travel assistance, and more
Accessia Health: Financial assistance for insurance premiums, medication copays, travel, and other essential medical expenses to individuals with qualifying household income and diagnosed chronic medical conditions
The Assistance Fund: Disease-specific programs to help patients and families facing high medical out-of-pocket costs
Chive Charities: Supports underserved Veterans, first responders, and rare medical individuals and their families with grants for things such as therapy equipment, wheelchair-accessible vans, mobility items, and more
Caregiver Aid (NORD): Provides financial assistance to give caregivers a break to attend a conference, event, or have a day away from caregiving
The Family Caregiver Toolbox: Resources for caregivers
Kindly Human: Resource that provides 24/7 peer support for caregivers
Triage Health: Provides free education on the legal and practical issues related to navigating a chronic or serious medical condition
Therapeutic Modalities for Rare Disease
Therapeutic modalities are methods or tools that clinicians use to address a disease or condition. Below are some common therapies used for rare diseases. As a note, not all of the therapies below can cure a disease, and not all therapies are available for all diseases. A patient’s medical team can provide more information about therapies currently available for a specific disease.
Care Team

Physicians
There are many people that may be part of the clinical team for a patient with a rare disease. The team members may vary based on many factors, including what rare disease a patient has, any co-occurring conditions, patient age and health, and more. Below are a few of the physicians that may be part of a rare disease patient care team:
A primary care physician is the “regular doctor” that patients see, and may also be called a family doctor, internist, general practitioner, or pediatrician. This doctor is seen for regular check-ups or physicals, non-urgent health issues, managing chronic conditions, and helping with coordination and referrals to other providers on the patient’s medical team. The primary care physician is the first place that many patients go to with questions or problems, and then this physician can either answer the question or direct the patient to another specialist who is better equipped to help with that specific question or problem.
A medical geneticist (also referred to as a clinical geneticist) is a doctor that diagnoses and treats genetic conditions. This doctor has a lot of knowledge about genetic diseases, and is often heavily involved with the diagnosis and treatment plan for the disease.
Specialists are doctors who have special training in a specific area of medicine. Depending on the rare disease, a patient may see a specialist that has expert knowledge on that disease or that part of the body to help treat or manage related symptoms. For example, a neurologist is a specialist who focuses on the brain and nervous system. A neurologist may help a patient with movement issues.

Genetic Counseling
Genetic counselors work to help people understand complex genetic information and the options available for genetic testing. They are very knowledgeable resources for patients.
Genetic counselors may discuss many topics with patients, including:
- Basics of genetics (what genetic diseases are, how they occur, etc.)
- Risk for having a genetic disease
- Different types of genetic tests available (and what the tests show)
- Costs of the tests and what insurance covers
- Risk of passing conditions down to children
- Test results
- Specifics about a patient’s genetic disease
- Potential treatment options
- Referrals to relevant healthcare providers and advocacy/support groups
Genetic counselors cannot provide medical treatment themselves.
Both in-person and/or telehealth appointments may be offered for genetic counseling. Telehealth are virtual healthcare appointments that are often done through a video or phone call. The types of appointment available may depend on a patient’s location, availability, and insurance.

Other Clinicians and Team Members
In addition to various physicians and genetic counselors, there may be other people on a patient’s care team depending on the disease a patient has. Below are a few other members of the care team that may be part of the patient’s journey:
Nurse practitioners, or NPs, are nurses that have completed an advanced degree and clinical training beyond their initial registered nurse (RN) practice. They may be involved in primary and specialty care.
Physicians assistants, or PAs, are licensed clinicians who practice medicine in every specialty and setting, and may be involved in primary and specialty care.
Physical therapists, or PTs, focus on helping patients improve physical function, mobility, and strength. They look at a patient’s movement patterns and develop a plan to improve any movement dysfunctions. They help with things like flexibility, range of motion, coordination, balance, sensory processing issues, and more.
Occupational therapists, or OTs, focus on activities that impact daily life. OT’s look at a patient’s functional abilities and create a plan to improve the patient’s independence and quality of life. They may help with things like eating, getting dressed, work tasks, leisure activities, fine motor skills, and more.
Social workers are professionals that help patients and families deal with life challenges by offering counseling and support, as well as connecting people with resources such as food or childcare.
Care coordinators help manage a patient’s care by doing things like making sure all needed appointments are scheduled and helping communicate between patients/families and different clinicians on the patient’s medical team.
Clinical Trials Information
What is a clinical trial?
A clinical trial is a research study that tests a medical, surgical, or behavioral intervention on patient volunteers. Clinical trials can also be observational, when patients are carefully monitored but don’t receive an intervention.
Does everyone get an intervention?
Clinical trials have different protocols based on what they are testing. Protocols can also vary by what phase the clinical trial is in. A few options for treatment protocols are:
- Everyone gets the intervention
- Some people get the intervention, some people get standard of care
- Some people get the intervention, some people get a placebo
Placebo
A placebo is an inactive substance that is used so that patients don’t know if they got the intervention or not. This helps researchers see if the intervention itself is providing a therapeutic benefit, or if people just think it is.
Placebo studies can be either be single-blind studies, where the patient does not know what they are getting but the researcher does, or double-blind studies, where neither the patient nor the researchers working directly with patients know who got the intervention.
Pros: Great at testing efficacy
Cons: Some patients get no treatment
Standard of Care
Standard of care is the current best practice for treating a condition. This standard care has already been approved by the FDA and is the typical care that patients with this condition receive.
Standard of care varies based on the condition, but could include things like taking medications, following a strict diet, or other medical care.
Pros: Everyone gets some form of treatment
Cons: May be more difficult for researchers to test effectiveness; standard of care in one country may be different or better than in other countries
Are placebos going to be used in rare disease clinical trials?
While this can vary by trial, there are many reasons not to use placebos for rare disease clinical trials. First, for some rare diseases, there are no other treatments, so it may be unethical to withhold the therapy. Second, for rare diseases there is only a small patient population. If trials use a placebo, patients may be less likely to join, therefore making the already small population to find participants from even smaller. Third, for some clinical trials, such as one testing a gene therapy, the procedures for getting gene therapy can be taxing and require risks, calling in to question whether using a placebo is ethical.

So if researchers don’t use placebos, what can they use instead to test effectiveness?
Researchers and governing bodies are still learning how to do this. One option is looking at biomarkers or patient’s status before, during, and after the trial. Another option could be comparing the therapy to current standard of care, or creating a “placebo group” using real-world data from others who have or have had the disease.
Is there anyone outside of researchers that monitor clinical trials?
Yes, there are groups that oversee the science aspects of clinical trials to check for safety and make sure trials are doing what they are supposed to do. Below are some groups that oversee U.S. clinical trials:
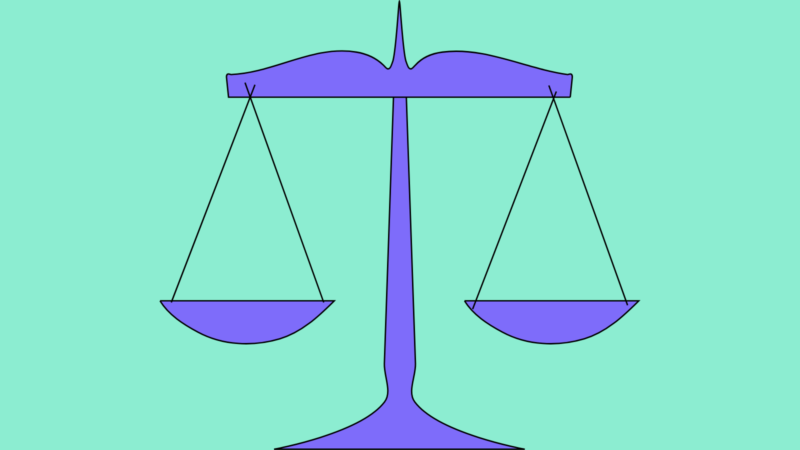
- Institutional Review Board: Approves and monitors trials to see that the potential benefits outweigh potential risks; ensures clinical trials are ethical and protects patient rights
- Office for Human Research Protections (OHRP): Helps protect the rights, welfare, and well-being of study participants
- Data and Safety Monitoring Board: Reviews data to look at differences between groups, safety issues, etc.
- FDA: Makes sure studies are following their protocols, provides guidance to researchers
Trial Phases
There are typically four phases for a clinical trial. Different phases of the clinical trial have slightly different purposes. Check out the diagram below to learn more.
As a note, not all clinical trials go through all four phases. There are many reasons a clinical trial may end at an earlier phase. Those reasons include (but are not limited to): safety concerns, the treatment not being effective, not enough funding to continue a trial, not getting enough patients to join and stay in trials, and regulatory issues.
***Phase I/II: Sometimes, phases I and II may be combined into one trial that looks at safety, dosing, and side effects. One reason these two phases may be combined is to use fewer participants, which may be beneficial in diseases with a small patient population.
Informed Consent
Informed consent is the process of learning about a trial, including learning its’ purpose, how long it lasts, what procedures or tests are done, risks, and benefits. Participants are given an informed consent document that they need to sign that says they understand the trial and agree to participate.
For children under 18 years old, a parent or guardian almost always has to consent to the trial. In addition, children over 7 years old are often asked if they agree to participate.
While the informed consent document is signed before a person starts partaking in a clinical trial, researchers should continue to provide information to participants throughout the entire study
Who pays for clinical trials?
Costs for clinical trials can be covered by the research team, health insurance, or out-of-pocket by patients. Before joining a clinical trial, it’s important to ask about different costs and determine who is responsible for what.
Costs could include (but are not limited to):
- Pre-intervention visits with researchers
- Pre-intervention care or preparation
- The intervention itself
- Post-intervention and follow-up care
- Travel to research or care facilities
- Lodging

How do people find clinical trials?
Clinicaltrials.gov
- Information about clinical trials around the world
- https://clinicaltrials.gov/
SCGE Gene Therapy Clinical Trial Browser
- For gene therapy clinical trials
- https://scge.mcw.edu/platform/data/search/ClinicalTrial
ResearchMatch
- Nonprofit that connects people interested in research studies with researchers at top medical centers across the US.
- https://www.researchmatch.org/
Which clinician on a medical team can a patient talk to about a clinical trial?
A patient’s medical geneticist (or medical genetics team) is a great resource for clinical trial information, because that is such a big part of their work. Patients can also talk to a primary care physician or specialist for input from different team members.
Getting Involved in Research
Why is it important to have patients and caregivers involved in rare disease research?
Patients and caregivers provide a unique perspective to research because they are the ones living with the disease. With this input, researchers can create therapies and clinical trials that best meet patient needs and address patient concerns. While participating in this early research does not give therapeutic benefit to the patient, it can provide hope and help patients and caregivers feel like they are doing something positive.
Involvement in clinical trial design
Patients can provide a lot of useful input in helping researchers design clinical trials before the trials begin. A few things that patients can provide information on are:
- How the study is designed
- What outcomes to measure for trial participants
- Improving educational materials and informed consent forms for potential trial participants so they are easier for patients to understand
- Helping with enrolling patients in trials
What is preclinical research?
Preclinical research is the early stage of research where scientists are studying a drug, therapy, or device before it is used in people. This is to do initial testing to see if they think the product is safe and works how it is supposed to.
Involvement in preclinical research
Patients and caregivers can help in preclinical research by sharing their stories and experiences with the researchers. This helps researchers focus on creating therapies to address symptoms or outcomes that are important to patients.
How do patients get involved in research?
A great way to get involved in research is through patient advocacy organizations for a specific rare disease.