2026
2025
 SCGE Researchers Create a Personalized Gene Editing Therapy to Treat a Rare Genetic Disease
SCGE Researchers Create a Personalized Gene Editing Therapy to Treat a Rare Genetic Disease
A team of SCGE researchers created a patient-specific gene editing therapy to treat an inborn error of metabolism in an infant. This groundbreaking research was led by Drs. Rebecca Ahrens-Nicklas (CHOP) and Kiran Musunuru (Penn).
 Meet the Expert: James Valentine and Richard Lewis
Meet the Expert: James Valentine and Richard Lewis
In this video of the Meet the Expert Series, panelists use their legal and U.S. Food and Drug Administration (FDA) expertise to provide practical guidance to rare disease researchers.
 Base Editing in Rare Diseases
Base Editing in Rare Diseases
In a scientific first, SCGE researchers supported used base editing to change single DNA bases and disrupt the repetitive sequence mutation and applied this technique in laboratory models of Huntington’s disease and Friedreich’s ataxia.
 Meet the Expert: Nick Buss, Ilan Irony, Allen Callaway
Meet the Expert: Nick Buss, Ilan Irony, Allen Callaway
In this video of the Meet the Expert Series, panelists discuss non-clinical study design and regulatory insights for a seamless transition.
Meet the Expert: Deanna Portero and Stefano Benvenuti
 In this video of the Meet the Expert Series, Deanna Portero and Stefano Benvenuti discuss alternative business models for non-commercially viable diseases.
In this video of the Meet the Expert Series, Deanna Portero and Stefano Benvenuti discuss alternative business models for non-commercially viable diseases.
SCGE Fall 2025 Meeting
 The SCGE Consortium met for an in-person meeting in Arlington, VA. The event was full of great presentations and insightful discussions. Check out a photo recap of the event below.
The SCGE Consortium met for an in-person meeting in Arlington, VA. The event was full of great presentations and insightful discussions. Check out a photo recap of the event below.
SCGE Releases Gene Therapy Trial Browser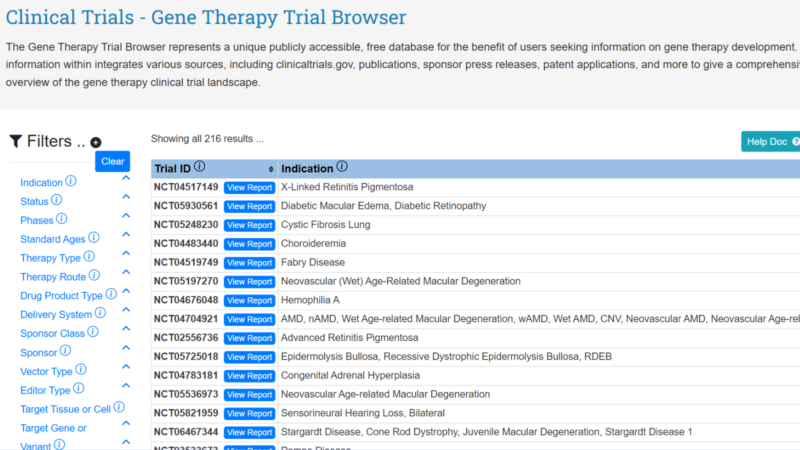
The SCGE has created a Gene Therapy Clinical Trial Browser. This publicly accessible, free database was created for the benefit of users seeking information on gene therapy development. The information within integrates various sources to give a comprehensive overview of the gene therapy clinical trial landscape.
NIH TARGETED Challenge Phase 2 Winners Announced
The winners of Phase 2 of the NIH TARGETED Challenge were recently announced. The TARGETED Challenge is a competition to improve technologies for delivering genome editing tools to cells in the body.
SCGE Spring 2025 Meeting
The SCGE Consortium met for an in-person meeting in Phoenix, AZ. The event was full of great presentations and discussions. Check out a photo recap of the event at the link below.
Meet The Expert: Steve Gray and Kiran Musunuru
In the second video of the Meet the Expert Webinar Series, Drs. Steve Gray and Kiran Musunuru share their experience and tips for preparing an IND application for gene therapy.
2024
SCGE Meet the Researcher Video Series
The TCDC is creating a video series where we interview different members that are part of Phase 2 of the SCGE Consortium. You can find these videos on our website or on the SCGE YouTube channel.
Bespoke Gene Therapy Consortium (BGTC) Regulatory Playbook
The Bespoke Gene Therapy Consortium (BGTC) drew upon their consortium subject matter experts and the collective experience of the scientific community to create a playbook that aims to bring safe, effective, and transformative gene therapies to patients in need.

Meet the Expert: Peter Marks and P.J. Brooks
In the first video of the Meet the Expert Webinar Series, Drs. Peter Marks and P.J. Brooks discuss the current landscape of the gene therapy regulatory and approval process, and the vision for where this can go in the future.
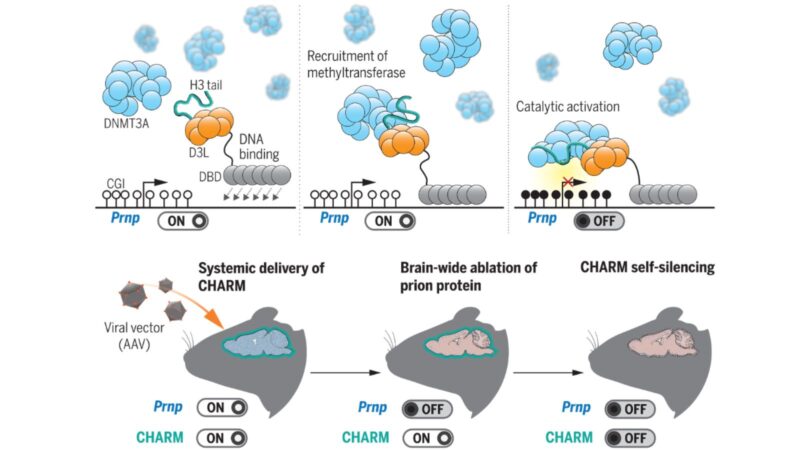
Brainwide silencing of prion protein by AAV-mediated delivery of an engineered compact epigenetic editor
SCGE researchers developed CHARM, a molecular-editing tool that’s small enough to be delivered to the brain to shut down the production of proteins that cause prion diseases.
 SCGE Fall 2024 Meeting Recap
SCGE Fall 2024 Meeting Recap
The fall 2024 SCGE Consortium in-person meeting was September 17th and 18th. The event was a great opportunity for consortium members to hear from each SCGE Phase 2 project and collaborate with other researchers. Check out some highlights from the meeting in this post.
 NIH Launches Phase 2 of the TARGETED Challenge
NIH Launches Phase 2 of the TARGETED Challenge
The National Institutes of Health (NIH) has launched the second phase of the Targeted Genome Editor Delivery (TARGETED) Challenge, a competition aimed at improving the state of in vivo delivery technologies for genome editors in two target areas. Participation in Phase 1 is not required to join Phase 2. More information is available on the Challenge website.
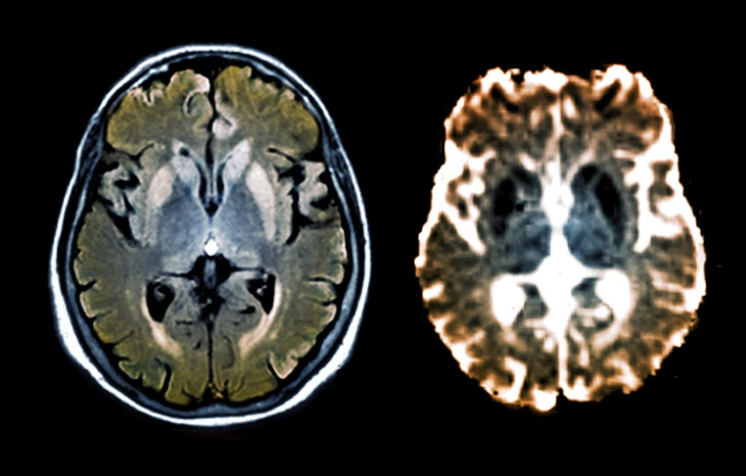
‘Epigenome editor’ silences gene that causes deadly brain disorders
SCGE researchers developed CHARM, a molecular-editing tool that’s small enough to be delivered to the brain to shut down the production of proteins that cause prion diseases.
New Notice of Funding Opportunity from The NIH Somatic Cell Genome Editing Program
The NIH Common Fund issued a new Notice of Funding Opportunity (NOFO) "Technologies and Assays for Therapeutic Genome Editing INDs (U01, Clinical Trial Not Allowed)" to support the second phase (FY2023-2027) of the Somatic Cell Genome Editing (SCGE) program. The purpose of this NOFO to support the optimization and evaluation of IND-enabling technologies and assays to help accelerate the clinical development and evaluation of novel somatic cell genome editing therapeutics to treat a broad array of rare and common diseases.
Applications are due on July 26, 2024, by 5:00 PM local time of applicant organization.
2023

TARGETED Challenge Phase 1 Winners Announced by NIH
The National Institutes of Health (NIH) recently announced the winners for Phase 1 of the TARGETED (Targeted Genome Editor Delivery) Challenge. In Phase 1, there were 54 proposals that outlined solutions to address one of two target areas: Programmable Delivery System for Gene Editing and Crossing the Blood-Brain Barrier. To see a full list of Phase 1 winners, click the button below.

A Closer Look at the CRISPR/Cas9 Gene Therapy for Sickle Cell Disease
The National Center for Advancing Translational Sciences (NCATS) researchers Dr. P.J. Brooks and Dr. Jeanita Pritchett Clay wrote an article discussing the impact of this discovery on the African American community as well as the potential to apply the approach to other diseases.

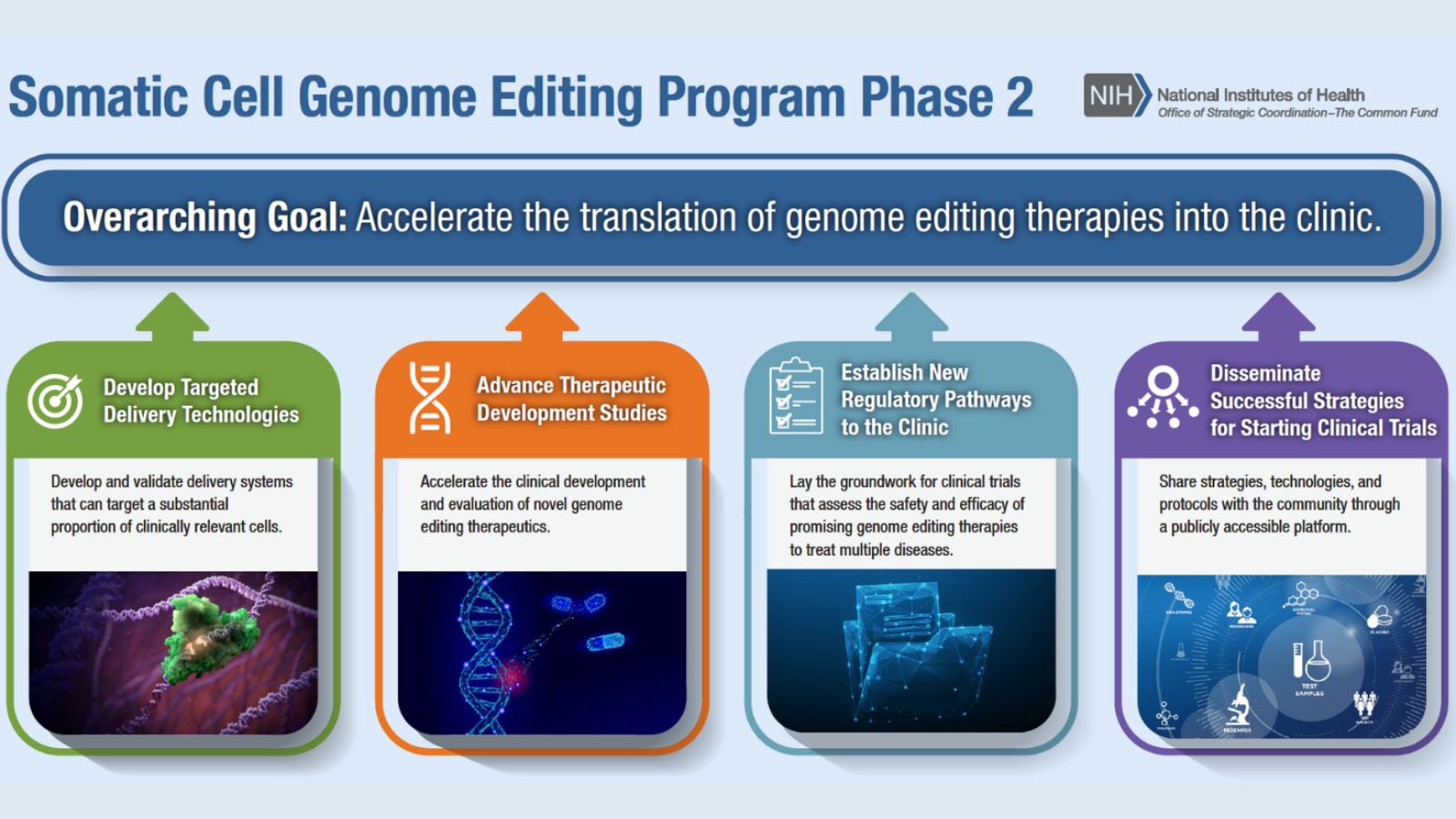
New Funding Opportunity to Support Phase 2 of the SCGE Program
The purpose of this new funding opportunity is to support IND-enabling studies for the development of a novel in vivo genome editing therapeutic platform (genome editor plus delivery system) for two or more disease indications, using the same genome editor, route of administration, and delivery system.
NIH Launches Phase 2 of the SCGE Program with a Series of New Awards
Join thousands of gene and cell therapy professionals for the ASGCT 26th Annual Meeting in Los Angeles, California, between May 16-20, 2023. Keynote speakers include Jennifer Doudna, PhD, a 2020 Nobel Prize winner, and David R. Liu, PhD, from the Broad Institute of Harvard and MIT.

NIH Launches the TARGETED Challenge
The National Institutes of Health (NIH) has launched the TARGETED (Targeted Genome Editor Delivery) Challenge to advance genome editing technology by sourcing innovative solutions for delivering genome editors to somatic cells. The Challenge is open to qualified groups or teams from organizations or institutions, particularly those in the genome editing or vehicle delivery fields, and will take place in three phases: Proposal, Preliminary Data, and Final Data, Independent Testing, and Validation.

American Society of Gene + Cell Therapy 26th Annual Meeting
Join thousands of gene and cell therapy professionals for the ASGCT 26th Annual Meeting in Los Angeles, California, between May 16-20, 2023. Keynote speakers include Jennifer Doudna, PhD, a 2020 Nobel Prize winner, and David R. Liu, PhD, from the Broad Institute of Harvard and MIT.
2022

Clinical Update: Graphite Bio Doses First Patient With Potential CRISPR Cure for Sickle Cell Disease
Graphic Bio, a biotechnology startup in San Francisco, dosed their first patient with nulabeglogene autogedtemcel (nula-cel), a CRISPR therapeutic strategy for treating sickle cell disease (SCD). The dose was performed as part of Phase 1/2 of the CEDAR trial, a multi-center, open-label study designed to evaluate the safety, efficacy and pharmacodynamics of the new therapeutic candidate in approximately 15 adult and adolescents with severe SCD.

The SCGE Consortium Launches Their Public-Facing Toolkit
The SCGE Consortium officially launched their public-facing Toolkit, a platform housing data generated by labs across all consortium initiatives. Users can explore and build upon both published and unpublished data from SCGE researchers such as validation of novel delivery systems and studies on the biological effects of genome editing. Check it out!

Verve Therapeutics Doses First Human with an Investigational In Vivo Base Editing Medicine, VERVE-101, as a Potential Treatment for Heterozygous Familial Hypercholesterolemia
Verve Therapeutics, a biotechnology company pioneering a new approach to the care of cardiovascular disease with single-course gene editing medicines, today announced that the first patient has been dosed with VERVE-101, in its heart-1 clinical trial.

Notice of Change to Application Due Date for RFA-RM-22-016 “Platform Clinical Trials of Genome Editors in Multiple Diseases (UG3/UH3, Clinical Trial Required)”
Key dates have changed for due dates for SCGE Phase 2 funding opportunity “Platform Clinical Trials of Genome Editors in Multiple Diseases (UG3/UH3, Clinical Trial Required).” LOIs are now due October 7, 2022 and full applications are due November 20, 2022.

Nobel Prize Co-Winner Dr. Jennifer Doudna Reflects on 10 Years of CRISPR
In 2020, Dr. Jennifer Doudna and Dr. Emmanuelle Charpentier received the Nobel Prize in Chemistry for their transformative work with CRISPR/Cas9 gene editing engineering. 10 years after CRISPR’s debut, Dr. Doudna interviews with STAT News to reflect on the future of CRISPR.

NIH Common Fund Announces Phase II of the SCGE
The NIH Common Fund has announced new funding opportunities to launch Phase II of the Somatic Cell Genome Editing (SCGE) Consortium. Phase II will aim to continue the consortium’s success in gene editing research and ultimately develop gene therapy treatments to treat genetic disorders.
SCGE Consortium Launches Nature Collection
The SCGE Consortium has launched their Nature Collection page highlighting publications on gene editing research and accomplishments made by consortium members.
2021

Jennifer Doudna’s Biotech Company Mammoth Bio Partners with Vertex Pharmaceuticals to Invest in Cas Enzymes for Gene Therapies
CRISPR pioneer Jennifer Doudna and her biotechnology company Mammoth Bio will receive up to $650 million in future payments to use Mammoth Bio’s ultracompact Cas enzymes for creating gene editing therapies.

SCGE Consortium Marker Paper Published in Nature
The NIH CommonFund and the SCGE Consortium is proud to present our published marker paper discussing the central goals and hopes for the consortium in advancing gene editing. The move from reading to writing the human genome offers new opportunities to improve human health. The United States National Institutes of Health (NIH) Somatic Cell Genome Editing (SCGE) Consortium aims to accelerate the development of safer and more-effective methods to edit the genomes of disease-relevant somatic cells in patients, even in tissues that are difficult to reach.
Francis Collins & David Liu Paper Accepted in Nature
Base-editing successfully treats Progeria in mice. Correcting the mutation that causes progeria with base editing leads to strong symptom reduction and longer lifespan in an animal model.
2020

Nobel Prize in Chemistry Awarded to Emmanuelle Charpentier and Jennifer A. Doudna
Emmanuelle Charpentier and Jennifer A. Doudna have discovered one of gene technology’s sharpest tools: the CRISPR/Cas9 genetic scissors. Using these, researchers can change the DNA of animals, plants and microorganisms with extremely high precision. This technology has had a revolutionary impact on the life sciences, is contributing to new cancer therapies and may make the dream of curing inherited diseases come true.
Methods Matter: Standard Production Platforms for Recombinant AAV Produce Chemically and Functionally Distinct Vectors
Different approaches are used in the production of recombinant adeno-associated virus (rAAV). The two leading approaches are transiently transfected human HEK293 cells and live baculovirus infection of Spodoptera frugiperda (Sf9) insect cells. Unexplained differences in vector performance have been seen clinically and preclinically.
mRNA Delivery Using Bioreducible Lipidoid Nanoparticles Facilitates Neural Differentiation of Human Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are widely used in regenerative medicine and tissue engineering and delivering biological molecules into MSCs has been used to control stem cell behavior.
CRISPR-CasΦ from huge phages is a hypercompact genome editor
Compact defense system in bacteriophages
The CRISPR-Cas system, naturally found in many prokaryotes, is widely used for genome editing. CRISPR arrays in the bacterial genome, derived from the genome of invading viruses, are used to generate a CRISPR RNA that guides the Cas enzyme to destroy repeat viral invaders. Recently, an unexpectedly compact CRISPR-Cas system was identified in huge bacteriophages.
2019
 Search-and-replace genome editing without double-strand breaks or donor DNA
Search-and-replace genome editing without double-strand breaks or donor DNA
10/21/2019 – Congratulations to the Lui Group on the publishing of their exciting new article on prime editing which is a versatile and precise gene editing tool which was published today in Nature.
Please read the commentary by Heidi Ledford titled “Super-precise new CRISPR tool could tackle a plethora of genetic diseases” here.
 Mary E. Shimoyama, PhD, associate professor of biomedical engineering, passed away on Feb. 19. She was 67.
Mary E. Shimoyama, PhD, associate professor of biomedical engineering, passed away on Feb. 19. She was 67.
Dr. Shimoyama has been involved with MCW’s Rat Genome Database since she first began working here in 2000, helping it evolve into the leading resource for rat genomic, genetic, phenotype and disease data. She earned her PhD in biomedical informatics from the University of Wisconsin-Milwaukee in 2011 and became an assistant professor of surgery at MCW. In 2016, she was promoted to associate professor in the Marquette University and MCW Joint Department of Biomedical Engineering. Dr. Shimoyama was exceptionally, and justifiably, proud of becoming the principal investigator for the Rat Genome Database and leading it through a recent competitive renewal. She was also honored to be the Vice Chair of Research and Clinical Affairs for the department this past year.
 NIH Awards $89 Million for Additional Projects to Advance Genome Editing
NIH Awards $89 Million for Additional Projects to Advance Genome Editing
10/1/2019 – Genome editing is a promising technology that could offer new treatments or cures for diseases, but challenges remain. To help address them, the National Institutes of Health (NIH) has awarded 24 additional grants to researchers across the United States and Canada through the Somatic Cell Genome Editing (SCGE) Program.
Nano-Sized Solution for Efficient and Versatile CRISPR Gene Editing
9/17/2019 – If used to make non-heritable genetic changes, CRISPR gene-editing technology holds tremendous promise for treating or curing a wide range of devastating disorders, including sickle cell disease, vision loss, and muscular dystrophy. Early efforts to deliver CRISPR-based therapies to affected tissues in a patient’s body typically have involved packing the gene-editing tools into viral vectors, which may cause unwanted immune reactions and other adverse effects.
 NIH supports international moratorium on clinical application of germline editing
NIH supports international moratorium on clinical application of germline editing
3/13/2019 – Today, leading scientists and ethicists from seven countries have called for an international moratorium on the use of genetic editing to modify the human germline for clinical purposes. The call comes in the wake of irresponsible and unethical research in China, in which twins were born after alterations to their DNA before implantation. This unexpected and unwelcome revelation roiled the scientific community and the general public, and crystalized the need for guiding international principles. Research on the potential to alter the very biological essence of humanity raises profound safety, ethical, and philosophical issues.